the energy band in which free electrons exist is the|Band Theory of Solids : Tagatay The valence electrons are so loosely attached to the nucleus that even at room temperature, few of the valence electrons leave the band to be free. These are called .
🍀 Minimum deposit: £10: 🍀 Withdrawal limits: Depends on the withdrawal policy: 🍀 Payment options: Visa, MasterCard, Paypal, Apple Pay, Neteller & More . . Well you have come to the right place! At 888 casino, we have so many exciting slot games with extraordinarily big Jackpots! In addition we offer an incredible variety of Daily .
the energy band in which free electrons exist is the,The electrons of a single free-standing atom occupy atomic orbitals, which form a discrete set of energy levels.The allowed energy levels in an . The energy band that consists of free electrons energy levels, is known as the conduction band. For electrons to be free, external energy must be applied such .The energy band in which free electrons exist is the: valence band conduction band first band inner shells second band. This problem has been solved! You'll get a detailed .energy bands are separated by energy bands (allowed energy levels) energy gaps or band gaps (region in energy for which no wavelike electron orbital exist) 4.1 Nearly .
OpenStax. Learning Objectives. By the end of this section, you will be able to: Describe two main approaches to determining the energy levels of an electron in a .The valence electrons are so loosely attached to the nucleus that even at room temperature, few of the valence electrons leave the band to be free. These are called .The highest energy band that is filled is known as a valence band. The next available band in the energy structure is known as a conduction band. In a conductor, the highest . Valence band. Forbidden Energy Gap. Conduction Band. Energy Band Theory. Valence Band. In solids, different ranges of energies can be present at complete zero temperature and the band which can .
Valence band. Forbidden energy gap. Conduction band. Valance Band. The flow of electrons within the atoms in fixed energy levels however the energy of the electron in the inner shell is superior to the outer shell of .Band Theory of Solids The energy band in which free electrons exist is the. conduction band. In a semiconductor crystal, the atoms are held together by. 1) the interaction of valence electrons 2)forces of attraction 3)covalent bonds. The atomic number of silicon is. 14. The atomic number of germanium is. 32.

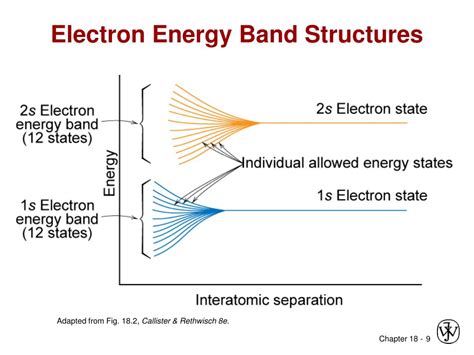
The existence of electron energy bands in solids makes it possible to understand this remarkable span.\(^{[1]}\) We can begin by considering the energy levels of the individual atoms as they are brought together. .Solution. Verified by Toppr. In a semiconductor, there are two types of charge carriers i.e. holes and electrons. Holes are present in the valence band, electrons are present in the conduction band. More the electrons in the conduction band, more is the conductivity of a semiconductor. Was this answer helpful?Since. Transcribed Image Text: 1) The energy band in which free electrons exist is band. 2) The electron energy (E) in the fifth orbit is.. Joule 3) Fermi-Dirac function is used to. 4) The momentum of the electron in the third orbit is. 5) At zero kelvin, the semiconductor becomes..Detailed Solution. Conduction band: The region in which free electrons remain is called the conduction band. In the case of conductors like metals, the valence band and conduction band overlap each other and almost all the charge carriers are found in the conduction band; This is the reason they are good conductors of electricity.
Electrical Engineering. The energy band in which free electrons exist is the conduction band O valence band Second band O first band * The load voltage and diode power of the following circuit are RTH 2 kQ 2-D APPROXIMATION VTH R 12 V I. 3.77V 2.64mA O 10v10w 12V 10mW 377V, 2,64mw.
The number of electrons remains the same and they always try to minimise the total energy. Now without any external energy, they’ll all be in the lowest energy state and no free electrons exist. Now of external energy is provided, the electrons have a tendency to use that energy to go to higher states.
the energy band in which free electrons exist is theThe free electrons’ availability at room temperature is huge. The energy band diagram of the conductor is shown below. energy-band-in-conductors. The main characteristics of conductors mainly include the energy gap like forbidden will not exist. The energy bands like valance as well as conduction will get overlapped.Step 1: We know that electrons exist in energy bands within a material. Step 2/5 Step 2: The valence band is the highest energy band that is filled with electrons at absolute zero temperature. Step 3/5 Step 3: The conduction band is the energy band above the valence band, where electrons can move freely and contribute to electrical conduction .The energy band in which free electrons exist is the (b) second band (c) conduction band (d) valence band (a) first band. C. conduction band. See an expert-written answer! We have an expert-written solution to this problem! 9. In a semiconductor crystal, the atoms are held together by (b) forces of attraction (a) the interaction of valence .
The energy of electrons in these bands will be different.The difference in energies of valence band and conduction band determines whether the solid is a conductor, semi - conductor or insulator .In a solid, the same principles apply. If N valence electron atomic orbitals, all of the same energy, are taken and combined to form bonds, N possible energy levels will result. Of these, N /2 will be lowered in energy and N .
It is the electronic energy band where there is no electron state exists due to quantization energy. The band obtained by separating conduction band and valence band is called as forbidden energy band or forbidden gap. .Semiconductors are classified by the fully occupied valence band and unoccupied conduction band. With the small band gap in between these two bands, it takes a certain amount of energy to excite the electrons from the valence to conduction band. Thus it follows that the higher the temperature, the more conductive the solid will be ( Figure 1).
Find step-by-step Engineering solutions and your answer to the following textbook question: The energy band in which free electrons exist is the (a) first band (b) second band (c) conduction band (d) valence band.The energy band in which free electrons exist is the Select one: A. second band e B. valence band C. first band D. conduction band This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The valence band refers to the energy level in an atom that contains the outermost electrons. It is the highest energy band filled with electrons at absolute zero temperature. Due to their proximity to the nucleus and other nearby atoms, electrons in the valence band are involved in the bonding interactions, such as covalent, ionic, or . The energy band in which free electrons exist is the (a) first band (b) second band (c) conduction band (d) valence band. View Answer: Answer: Option C. Solution: 9. In a semiconductor crystal, the atoms are held together by (a) the interaction of valence electrons (b) forces of attraction
the energy band in which free electrons exist is the|Band Theory of Solids
PH0 · [Solved] The energy band in which free electrons exist is
PH1 · What is Energy Band : Band Theory and Different Types
PH2 · What is Energy Band : Band Theory and Different
PH3 · Solved The energy band in which free electrons exist is the
PH4 · Lecture 4
PH5 · Energy bands in solids and their calculations
PH6 · Energy Band : Theory, Different Types and Its Properties
PH7 · Basic Electronics
PH8 · Band Theory of Solids
PH9 · Band Theory Of Solids
PH10 · 9.6: Band Theory of Solids
PH11 · 9.5 Band Theory of Solids